Abstract
INTRODUCTION
The validity of progression-free survival (PFS) as a clinical endpoint in multiple myeloma (MM) has been debated, largely based on the potential association between PFS and overall survival (OS). However, MM progression can cause morbid end-organ damage. Bone lesions (B) and renal dysfunction (R) may be irreversible, while progressive anemia (A) and hypercalcemia (C), though reversible, can cause severe symptoms, and all are sources of both morbidity and mortality. There is limited research on the rates of progression or on the OS implications associated with morbid progression events compared with asymptomatic biochemical progression. We therefore aimed to characterize the presence of CRAB symptoms at the time of progression and to examine the associations between morbid relapses and OS in 2 large, randomized clinical trials in transplant-ineligible elderly patients (pts) with MM that relapsed after initial therapy.
METHODS
This analysis used pooled data from the phase 3, randomized MM-020 and MM-015 trials of pts with newly diagnosed MM (NDMM) aged ≥ 65 years and included pts who progressed on study. Treatment arms in MM-020 were lenalidomide-dexamethasone (Rd) until progressive disease (PD), Rd for 18 cycles, and melphalan-prednisone-thalidomide, and in MM-015 were melphalan-prednisone, melphalan-prednisone-lenalidomide (MPR), and MPR followed by lenalidomide maintenance. CRAB (Calcium > 11.5 mg/dL, Renal insufficiency: creatinine > 2 mg/dL or creatinine clearance [CrCl] decrease by 10 mL/min, Anemia: hemoglobin < 10 g/dL, and Bone lesions) criteria were analyzed within 1 month before or after PD, defined as an increase of 25% from the lowest response value in ≥ 1 of the following: serum M protein, urine M protein, free light chain levels, or bone marrow plasma cell percentage. Pts with PD and meeting ≥ 1 CRAB criterion were considered to have morbid PD; all other pts with PD were categorized as asymptomatic. Demographic and baseline characteristics are reported by relapse group (morbid PD vs asymptomatic PD). The association between morbid PD and PFS from the time of progression (PFS2) as well as OS was examined using an adjusted Cox regression analysis adjusted for baseline pt characteristics, including age, gender, International Staging System (ISS) stage, creatinine, randomized treatment, and anemia. Other covariates were assessed in the window of 1 cycle before or after PD. PFS2 and OS were measured from time of PD. Multivariable Cox regression models estimated the association between morbid PD and PFS and OS. Direct adjusted survival curves based on these models were used to estimate median OS and PFS.
RESULTS
Of the 2082 pts in the pooled intent-to-treat population of MM-020 (N = 1623) and MM-015 (N = 459), 1243 pts (60%) had PD in first-line treatment for NDMM. At the time of first progression 543 pts (44%) were considered to have morbid (≥ 1 CRAB criterion) PD and 700 pts (56%) had asymptomatic PD. Of the pts with morbid PD, 12 (2%) had hypercalcemia, 271 (50%) had elevated creatinine, 370 (68%) developed anemia, and 79 (15%) developed new bone lesions. Baseline pt characteristics were generally similar between the morbid and asymptomatic PD groups, with a median age of 74.0 yrs for both groups; 51% vs 53% were male and 41% vs 37% had ISS stage III disease, respectively. Hemoglobin < 10 g/dL was less common in the morbid PD group (50% vs 80%), whereas CrCl < 60 mL/min and lactic dehydrogenase > 200 U/L were more frequent in the pts with morbid vs asymptomatic PD (40% vs 26% and 20% vs 13%, respectively).
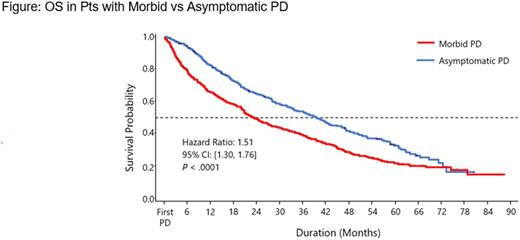
After adjustment for baseline characteristics, median PFS2 was 11.5 vs 20.0 mos in pts with morbid vs asymptomatic PD (HR = 1.56 [95% CI, 1.37-1.79]; P < .0001). Median OS was 23.2 mos in pts with morbid PD and 39.3 mos in pts with asymptomatic PD (HR = 1.51 [95% CI, 1.30-1.76]; P < .0001; Figure).
CONCLUSIONS
In this retrospective analysis of 2 large, randomized controlled trials, morbid PD was common, accounting for over 40% of progression events. Moreover, potentially irreversible events, worsening renal function and new bone lesions, occurred in 22% and 15% of pts, respectively. Finally, morbid progression was associated with shortened PFS2 and OS. Additional multivariate analyses examining the relationship between other prognostic factors (including cytogenetics and FISH) and morbid PD are ongoing.
Rosenberg: Amgen: Speakers Bureau. Facon: Amgen, Celgene: Speakers Bureau. Abouzaid: Celgene Corporation: Employment, Equity Ownership, Research Funding. Srinivasan: Celgene: Employment. Chung: Celgene Corporation: Employment, Equity Ownership. Parikh: Celgene Corporation: Employment. Tuscano: Takeda: Honoraria, Research Funding; Celgene Corporation: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Novartis Pharmaceuticals: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal